A COMPREHENSIVE QUANTITATIVE AND QUALITATIVE EVALUATION OF EXTRAPOLATION OF INTRAVENOUS PHARMACOKINETIC PARAMETERS FROM RAT, DOG, AND MONKEY TO HUMANS. I. CLEARANCE

By A Mystery Man Writer
This study was conducted to comprehensively survey the available literature on intravenous pharmacokinetic parameters in the rat, dog, monkey, and human, and to compare common methods for extrapolation of clearance, to identify the most appropriate species to use in pharmacokinetic lead optimization, and to ascertain whether adequate prospective measures of predictive success are currently available. One hundred three nonpeptide xenobiotics were identified with intravenous pharmacokinetic data in rat, dog, monkey, and human; both body weight- and hepatic blood flow-based methods were used for scaling of clearance. Allometric scaling approaches, particularly those using data from only two of the preclinical species, were less successful at predicting human clearance than methods based on clearance as a set fraction of liver blood flow from an individual species. Furthermore, commonly used prospective measures of allometric scaling success, including correlation coefficient and allometric exponent, failed to discriminate between successful and failed allometric predictions. In all instances, the monkey tended to provide the most qualitatively and quantitatively accurate predictions of human clearance and also afforded the least biased predictions compared with other species. Additionally, the availability of data from both common nonrodent species (dog and monkey) did not ensure enhanced predictive quality compared with having only monkey data. The observations in this investigation have major implications for pharmacokinetic lead optimization and for prediction of human clearance from in vivo preclinical data and support the continued use of nonhuman primates in preclinical pharmacokinetics.
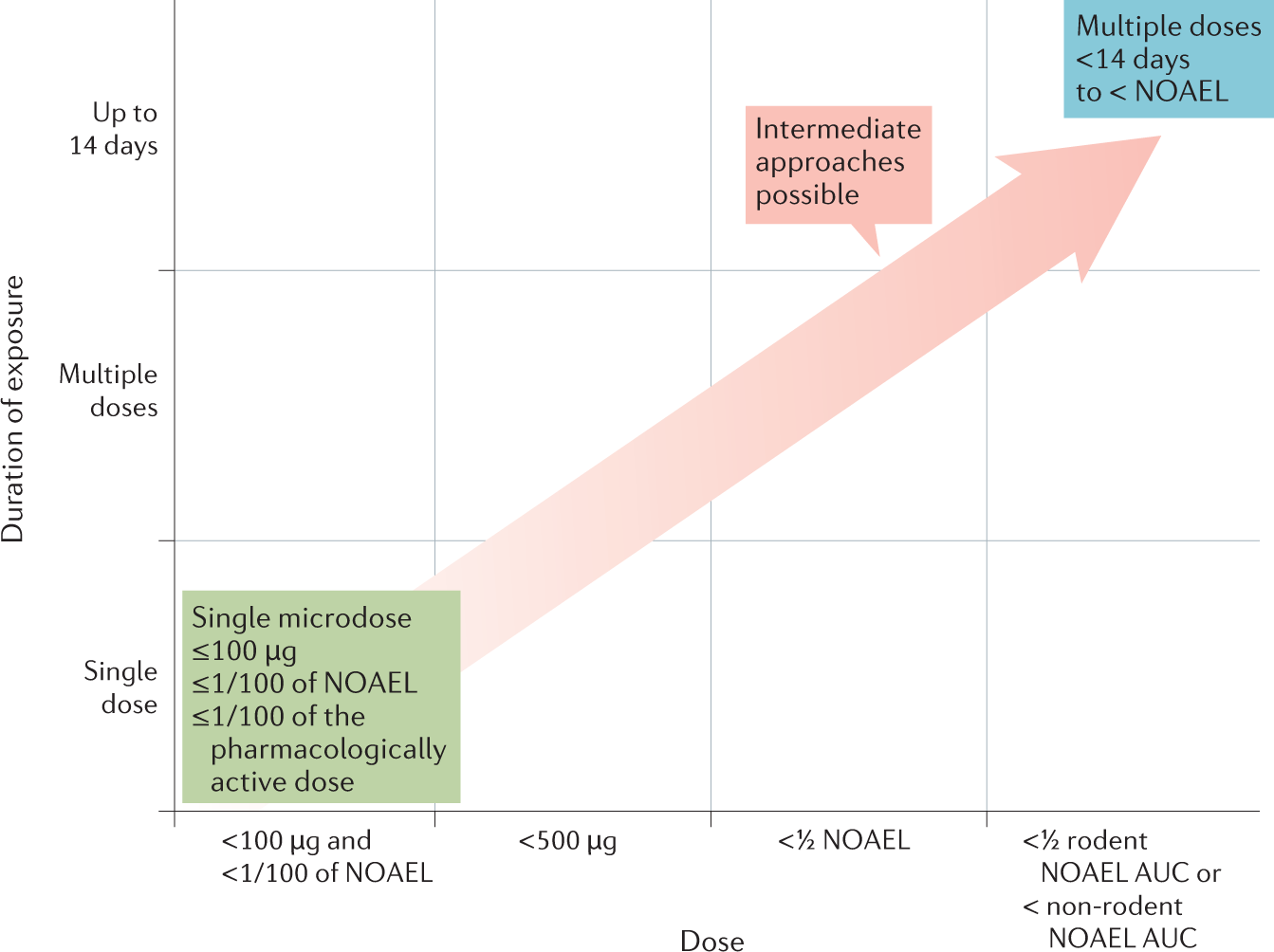
Phase 0/microdosing approaches: time for mainstream application in drug development?
Interspecies evaluation of a physiologically based pharmacokinetic model to predict the biodistribution dynamics of dendritic nanoparticles

Brian R Smith's research works GlaxoSmithKline, London (GSK) and other places
The TeGenero Incident and the Duff Report Conclusions: A Series of Unfortunate Events or an Avoidable Event? - Christopher J. Horvath, Mark N. Milton, 2009

In Silico Categorization of in Vivo Intrinsic Clearance Using Machine Learning
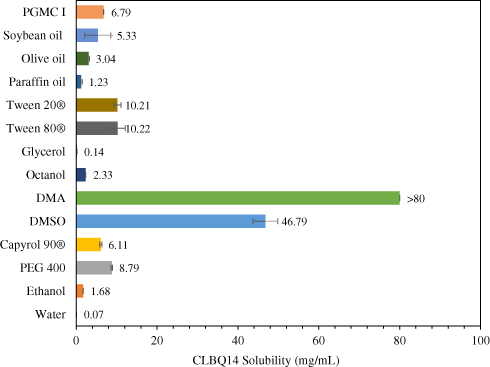
Pre-Clinical Pharmacokinetics, Tissue Distribution and Physicochemical

PDF) Open Flow Microperfusion as a Dermal Pharmacokinetic Approach to Evaluate Topical Bioequivalence

Interspecies allometric meta‐analysis of the comparative pharmacokinetics of 85 drugs across veterinary and laboratory animal species - Huang - 2015 - Journal of Veterinary Pharmacology and Therapeutics - Wiley Online Library

Keith W Ward's research works Reata Pharmaceuticals, Irving and other places
A novel strategy for physiologically based predictions of human pharmacokinetics - Document - Gale Academic OneFile
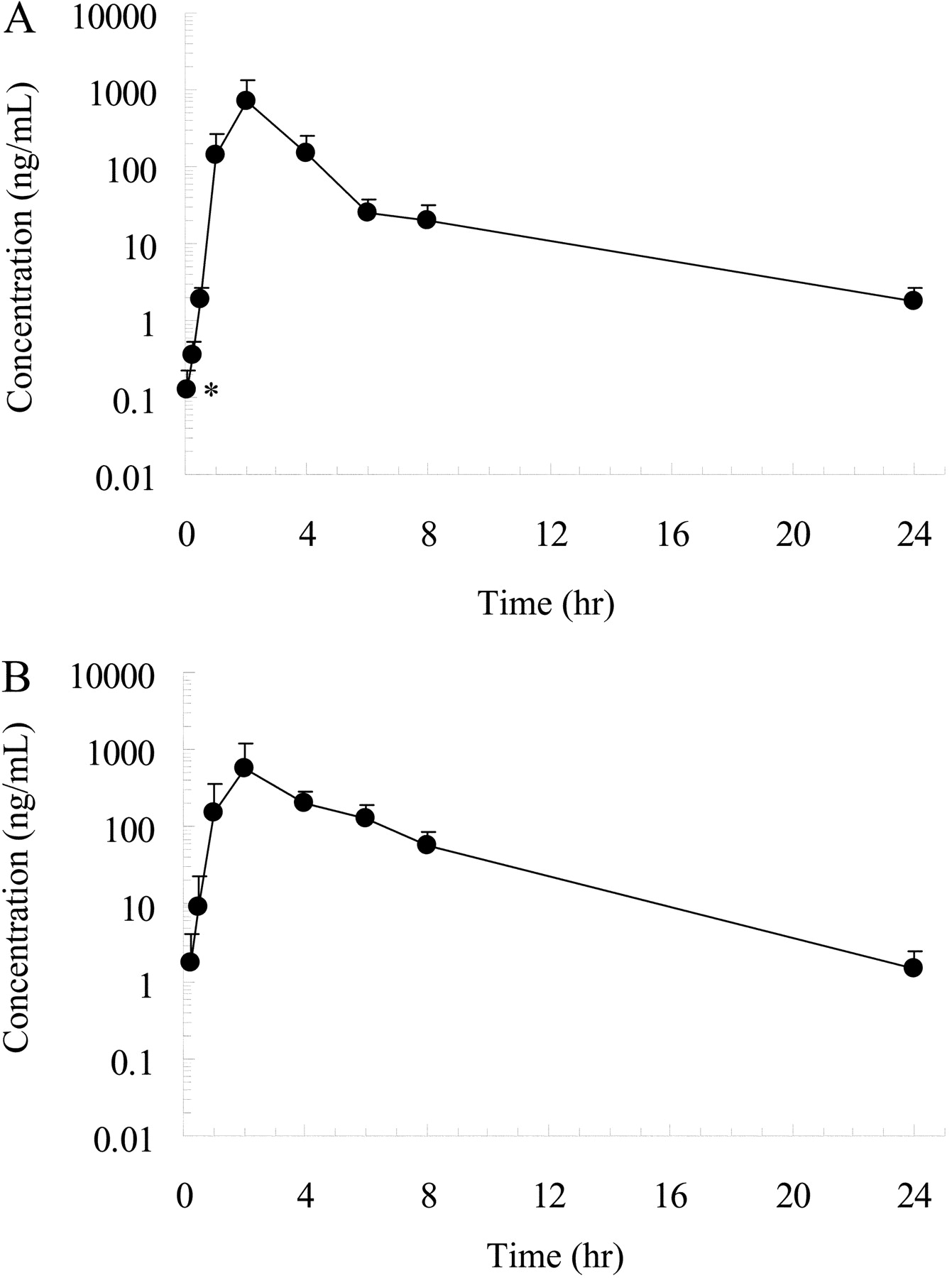
Effect of Oral Ketoconazole on Oral and Intravenous Pharmacokinetics of Simvastatin and Its Acid in Cynomolgus Monkeys
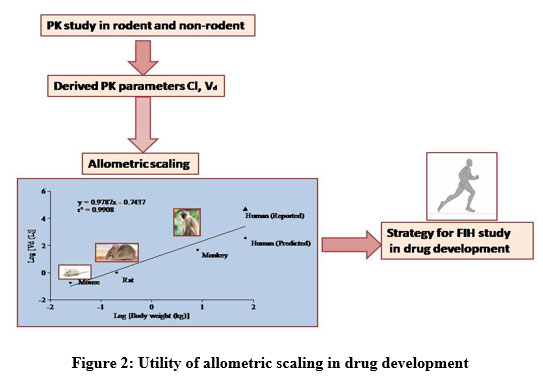
Advantages of Allometric Scaling Methods for Predicting Human Pharmacokinetics of Novel JAK Inhibitor -Baricitinib and Dose Extrapolation – Biomedical and Pharmacology Journal

Adenovirus Capsid-Based Anti-Cocaine Vaccine Prevents Cocaine from Binding to the Nonhuman Primate CNS Dopamine Transporter

PhRMA CPCDC Initiative on Predictive Models of Human Pharmacokinetics, Part 1: Goals, Properties of the Phrma Dataset, and Comparison with Literature Datasets - Journal of Pharmaceutical Sciences
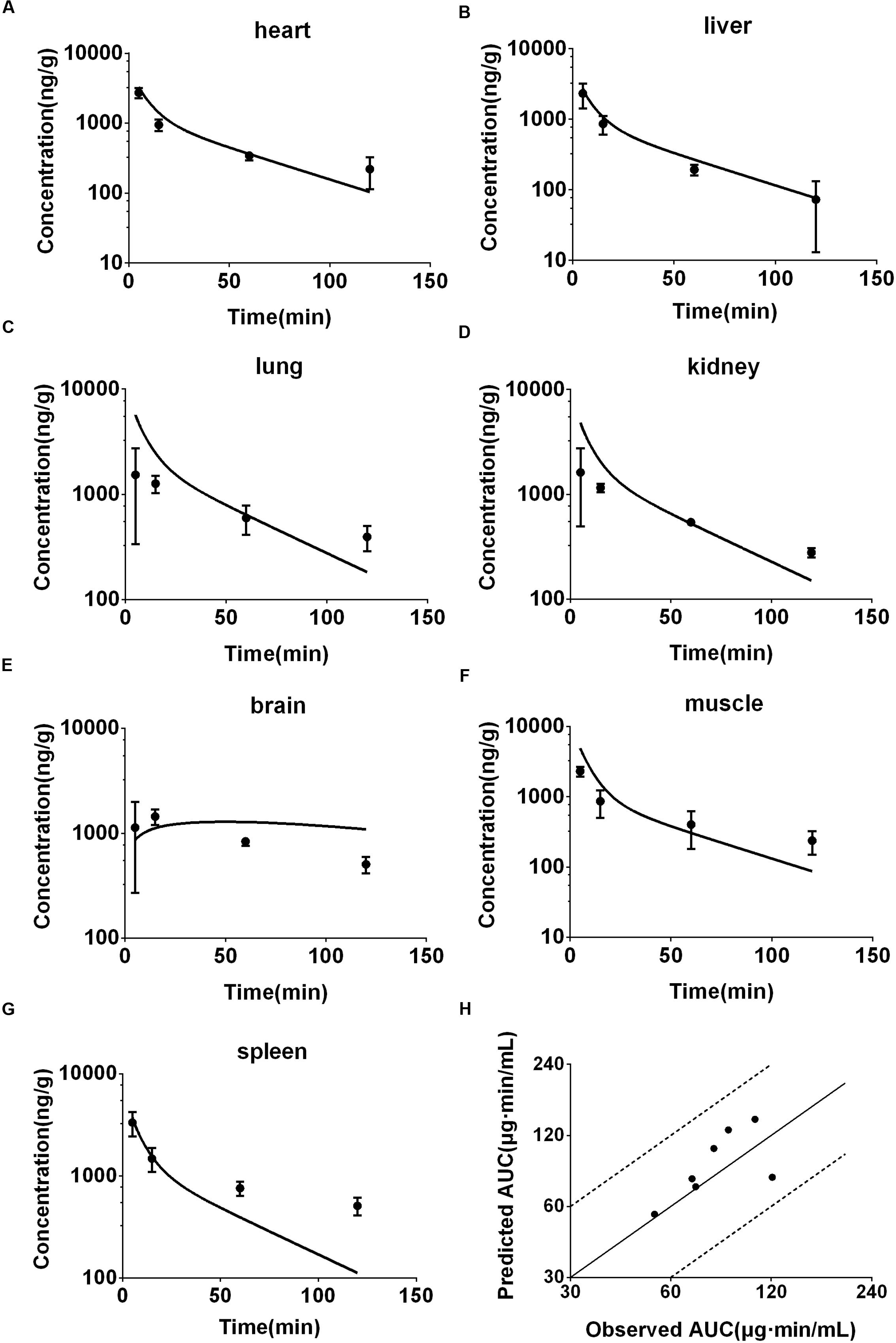
Frontiers Prediction of Deoxypodophyllotoxin Disposition in Mouse, Rat, Monkey, and Dog by Physiologically Based Pharmacokinetic Model and the Extrapolation to Human
- Mrat Clearance Bras for Women Pack of 6 Clearance Womens Embroidered Glossy Comfortable Breathable Bra Underwear No Underwire Nude Sports Bra L_17 Pink 38

- Mrat Ladies Down Gilets Clearance Sleeveless Winter Jackets for Women UK Zip Up Waistcoat Warm Short Bodywarmer Casual Solid Color Padded Gilet Windproof Thermal Sleeveless Coats Office Black XS : : Fashion

- Mrat Clearance Bras for Women Clearance Woman Summer Bra Without Underwire Vest Push up Bra Lingerie Underwear Chain Bra L_22 Orange 3XL

- Barrett MRAD - Wikipedia

- Mrat Summer Tee Shirts Womens 3/4 Sleeve Blouse Graphic Print T Shirt Half Button Up Shirt Crew Neck Tops Trendy Ladies Loose Fit Tunic Tops Casual Blouses Round Neck T Shirts Green

- Gaiam Premium Print Yoga Mat, Marrakesh, 5/6mm, Mats - Canada

- adidas Compression Calf Sleeve (Pack of 1) Compression calf sleeves, Calf sleeve, Adidas women

- Low-pass sequencing and imputation for evaluating genetic variation - Gencove

- Ross Plus Size Prom Dresses - UCenter Dress

- Gymshark Takki Tarjous - Recess Track Naisten Vaaleanvihreä
