SOLVED: How much will the temperature of a cup (180 g) of coffee at 95 ^ C be reduced when a 45 g silver spoon (specific heat 0.24 J / g^∘C )

By A Mystery Man Writer
VIDEO ANSWER: We are asked how much the temperature of a cup of coffee it is at 95 degrees celsius and we put in there a 45 gram silver spoon. The heat capacity is 0.24 joules per gram, degrees c. So here's my mass here's, my c and my initial
How much will the temperature of a cup (180 g) of coffee at 95 ^ C be reduced when a 45 g silver spoon (specific heat 0.24 J / g^∘C ) at 25^∘C is placed in the coffee and the two are allowed to reach the same temperature? Assume that the coffee has the same density and specific heat as water.
Numerade is a venture-backed, high-growth education technology startup based in Pasadena. We are singularly focused on creating exceptional video and interactive content experiences for education making the knowledge and skills of world class educators widely accessible and affordable to student audiences of all backgrounds. Our mission is to close the educational opportunity gap by unlocking and democratizing access to extraordinary educators and the content they have to offer.

Specific Heat Capacity
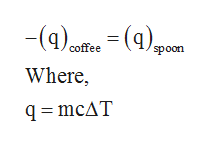
Answered: How much will the temperature of a cup…

SOLVED: A 69.0 g silver spoon at 25.5°C is placed in a cup of coffee at 91.1°C. How much heat does the spoon absorb from the coffee to reach a temperature of

Week 3 HW - Homework - CH. 5 Questions: 6. How much heat, in joules and in calories, must be added - Studocu

⏩SOLVED:How much will the temperature of a cup (180 g) of coffee at…

How much will the temperature of a cup (180 g) of coffee at
⏩SOLVED:How much will the temperature of a cup (180 g) of coffee at…
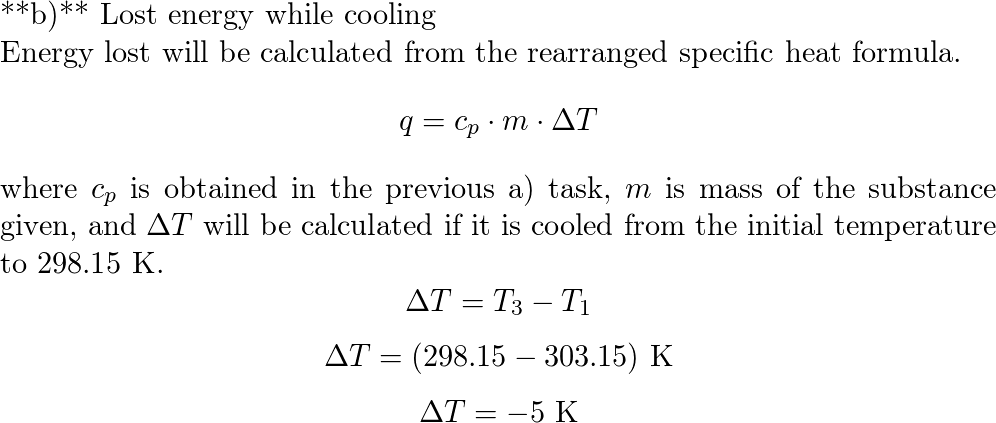

Answered: How much energy is needed to raise the…

5.2 Calorimetry
- Drakes Online Findon - Continental Cream Of Chicken Simmer Soup Packet 45g
- Gloria Flour - MEASURING YOUR INGREDIENTS IS THE MOST IMPORTANT
- Mama - 45g - Jok Cup - Instant Porridge Soup (Chicken)

- No Name Mini Cups - 1 ea Real Canadian Superstore

- Hostess Cup Cakes – Frosted Chocolate Cake with Cream Filling 45g






