Friday, Jul 05 2024
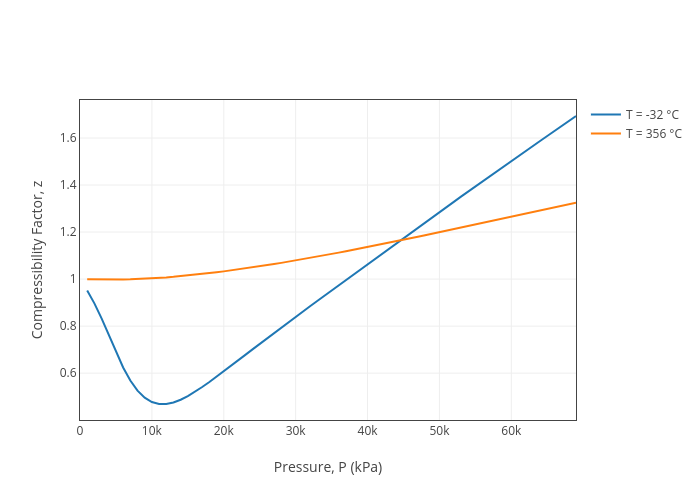
OneClass: For a real gas, the compressibility factor, Z, is

By A Mystery Man Writer

OneClass: 2. Fugacity for a van-der-Waals gas Let's get a feel for how much fugacity deviates from pr

Compressibility Factor Z // Thermodynamics - Class 85
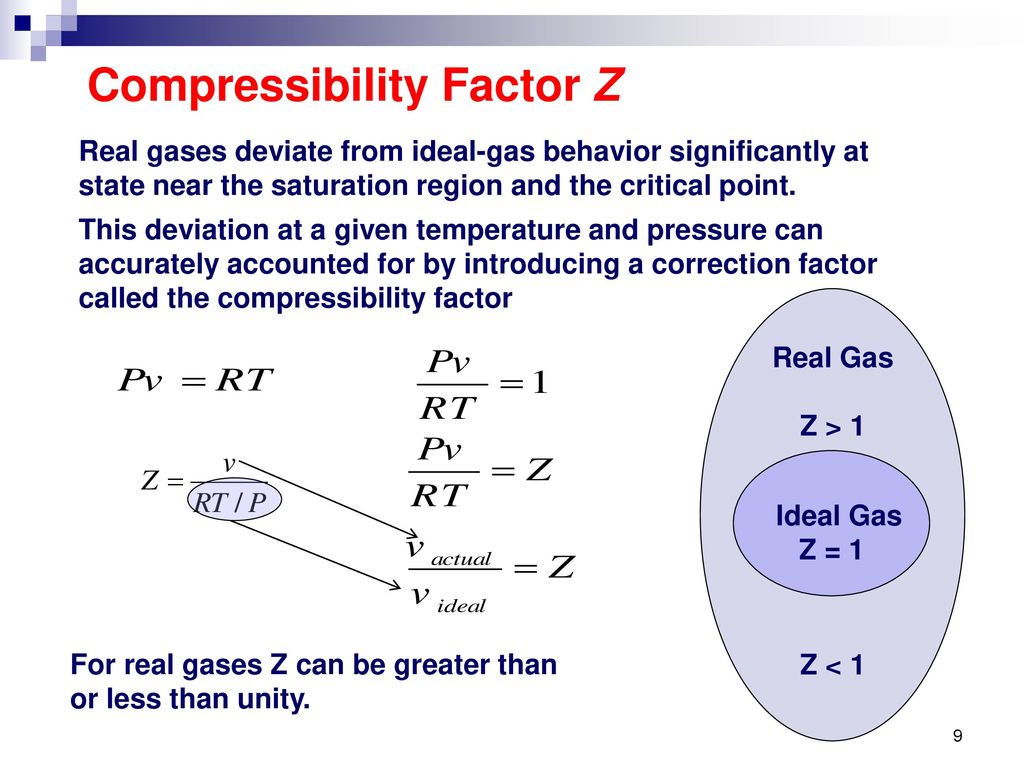
The Ideal Gas. - ppt download

Compressibility factor - Wikipedia

OneClass: For a real gas, the compressibility factor, Z, is defined as Z (T, P) = PV/nRT For an ideal

حرارة وديناميكا حرارية - ppt download

compressible flow related terms - Department of Mechanical and

Compressibility factor (z): real gases deviate from ideal behav-Turito

The compressiblity factor Z for 1 mole of a real gas at low pressure can be written as

where Z is the compressibility factor that

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as
Related searches
Related searches
©2016-2024, jazbmetafizik.com, Inc. or its affiliates