For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is?\n \n \n \n \n 1.$\\left( {1 + \\dfrac{b}{V}} \\right)$ 2.$\\left( {1 - \\dfrac{b}{V}} \\right)$3.$\\left( {1 + \\

By A Mystery Man Writer
For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is?\n \n \n \n \n 1.$\\left( {1 + \\dfrac{b}{V}} \\right)$ 2.$\\left( {1 - \\dfrac{b}{V}} \\right)$3.$\\left( {1 + \\dfrac{a}{{RTV}}} \\right)$4.$\\lef

UUVI 20. For Co. isothermal is of the as shown, near the point A compressibility factor Z is U co B ideal DV

Real Gas Behavior The Compression Factor (Z) [Example #2]

Compressibility factor - Wikipedia
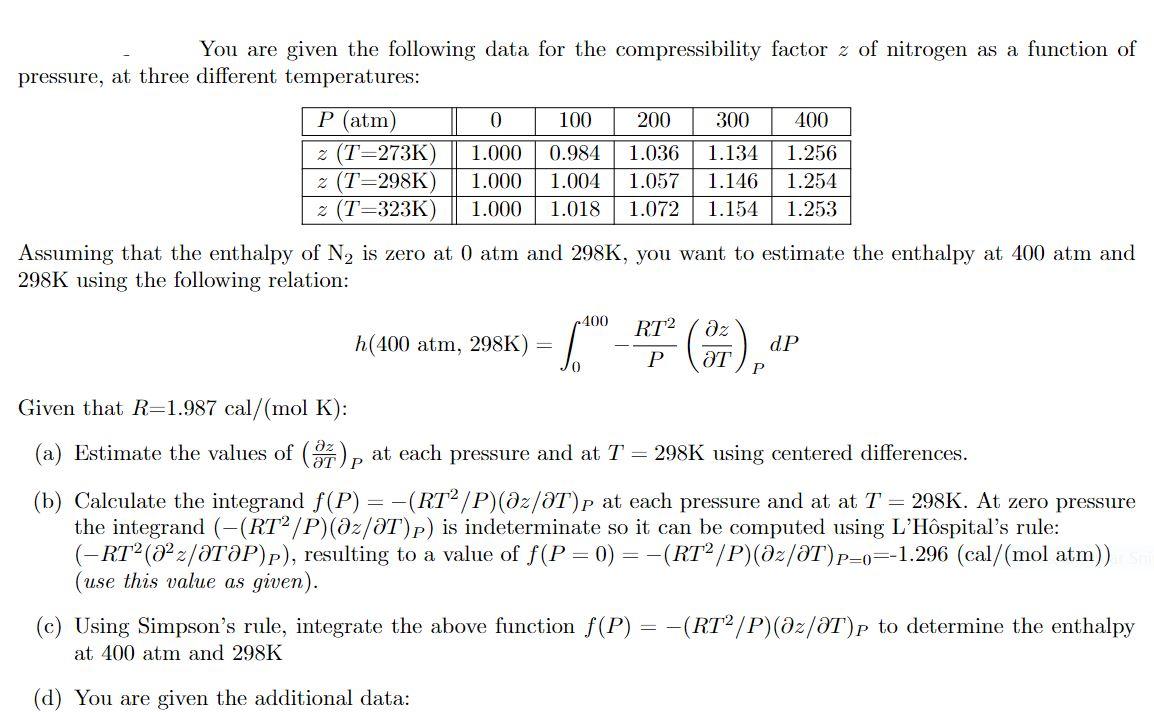
Solved You are given the following data for the

Assertion: Compressibility factor `(Z)` for non ideal gases is always greater than `1`.
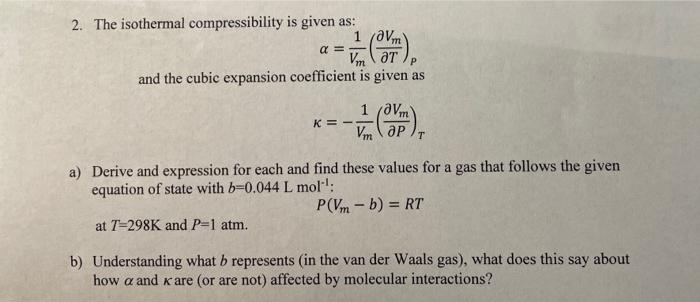
Solved 2. The isothermal compressibility is given as
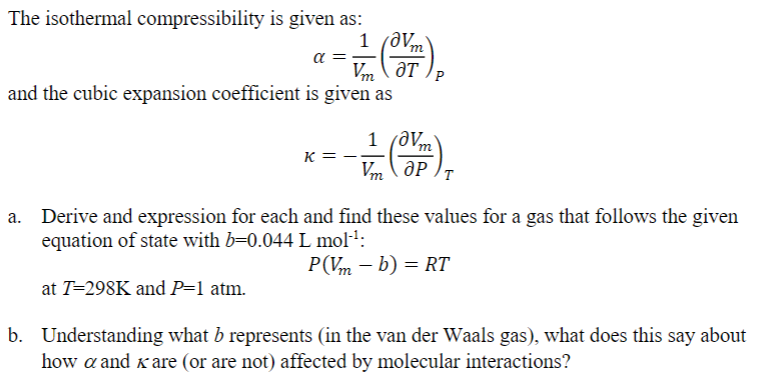
Solved The isothermal compressibility is given as
For CO, isotherm is of the type as shown: Near the point A, compr
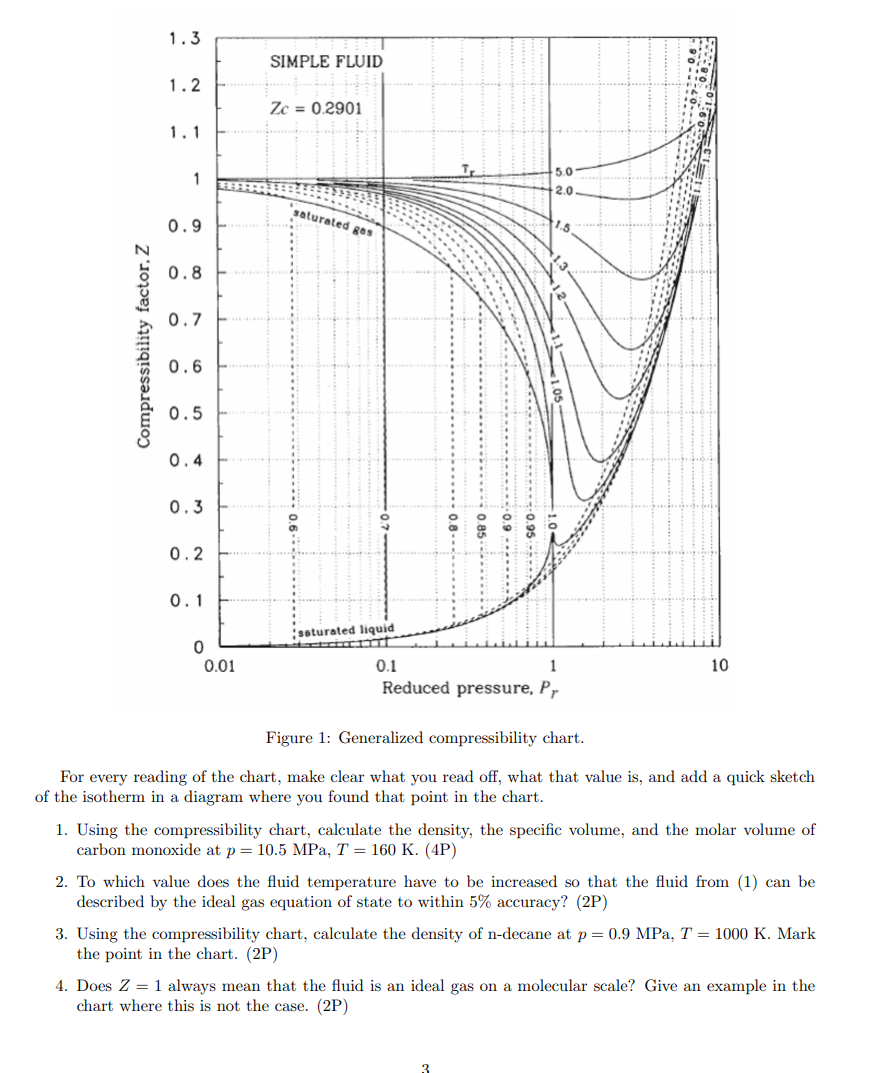
Solved 1.3 SIMPLE FLUID 1.2 Zc = 0.2901 1.1 1 5.0 2.0

Compressibility factor - Wikipedia

For CO, isotherm is of the type as shown in the figure.Near the point A ,compressibility factor Z will be.

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with Compressibility Factor

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with Compressibility Factor
- Air Compressibility Factor Table - EnggCyclopedia
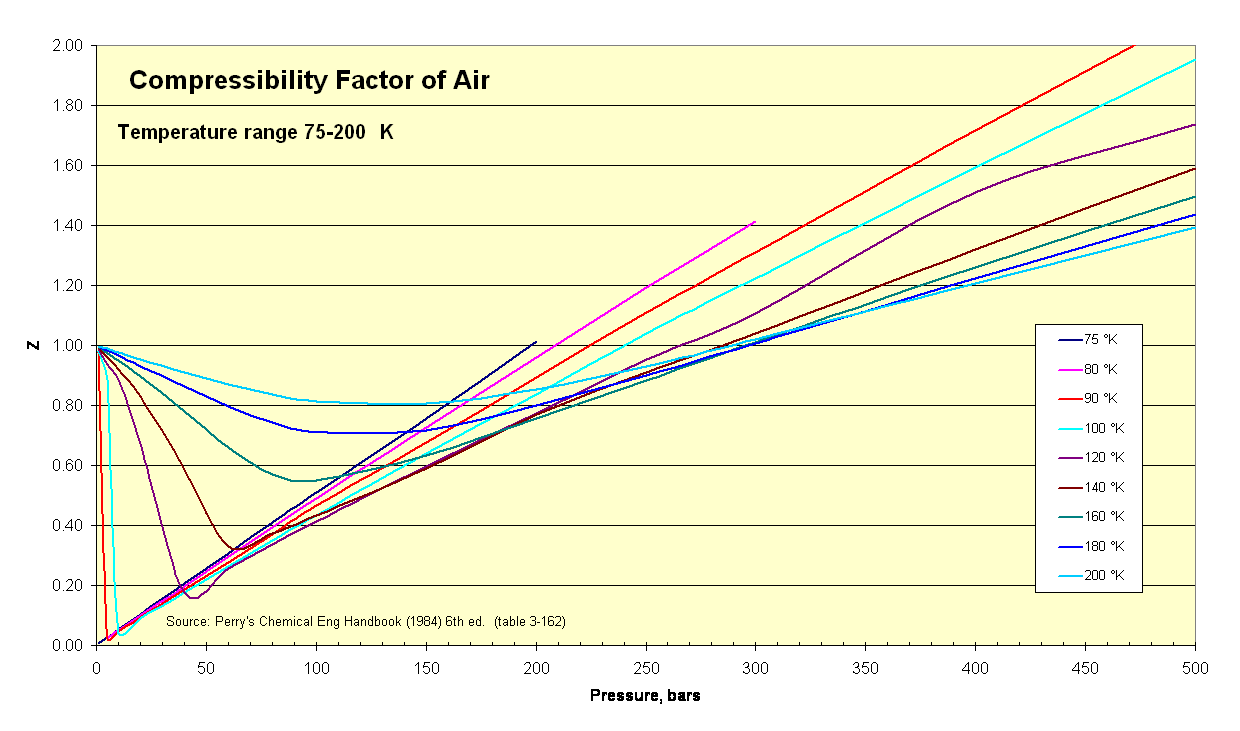
- e Compressibility factor (Z) for hydrogen WRT pressure and

- Compressibility Factor Charts
- Write the expression for the compressibility factor (Z) for one

- plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange

- Punto de cruz, cenefas para caminos de mesa o manteles!!!

- Calvin Klein Women 5-Pack Cotton Form Thong

- Lace Bra Black Calvin Klein Underwear - Women

- Fox & Royal Women's Plus Size Sophie Strappy Bralette - Black - 18w : Target
- Vgplay Red Bra Push Up Bras for Women Deep Plunge V Neck Lingerie Clear Straps Multiway


