The graph of compressibility factor (Z) vs. P for one mole of a real gas ..
By A Mystery Man Writer
Solution For The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following diagram. The graph is plotted at constant temperature 273K. If the slope of graph at very hi
The graph of compressibility factor (Z) vs. P for one mole of a real gas is shown in following diagram. The graph is plotted at constant temperature 273K. If the slope of graph at very high pressure (dPdZ) is (2.81)atm−1 , then calculate volume of one mole of real gas molecules (in L/mol) Given : NA=6×1023 and R=27322.4LatmK−1mol−1

The graph of compressibility factor (Z) :vs: P one mole of a real

If the slope of 'Z' (compressibility factor) vs. 'p' curve is constant

The compressibility factor (Z) vs P one mole of a real gas is
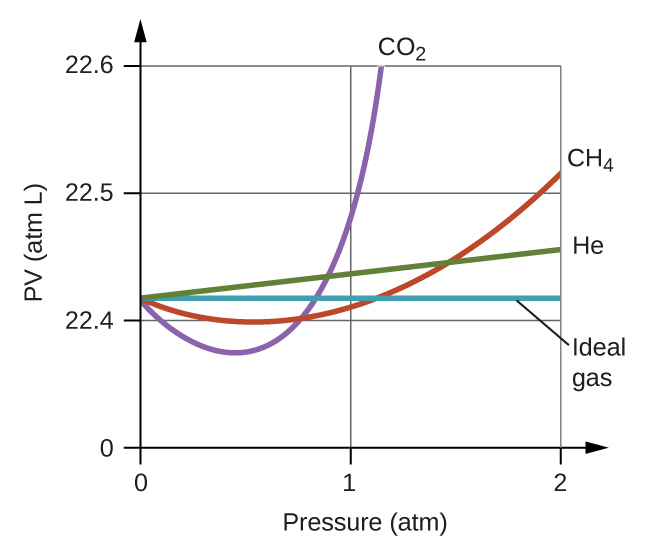
Non-Ideal Gas Behavior – Atoms First / OpenStax

Energies, Free Full-Text

Illustrations Illustration 1. 2 N2O5→4NO2+O2 यदि NO2 की
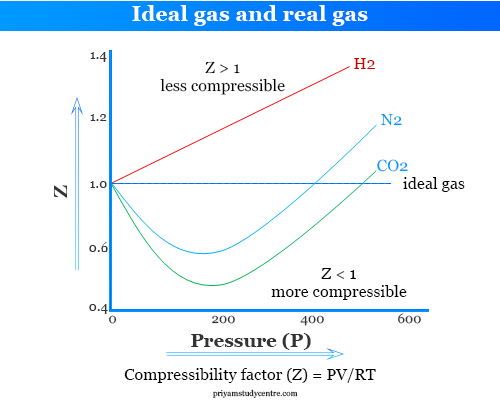
Ideal and Real Gases - Definition, Comparison, Properties

For one mole of a real gas, curves are plotted under different

The graph of compressibility factor (Z) v/s P 1 mol of a real gas

Deviation From Ideal Gas Behavior - Study Material for IIT JEE

Gaseous State, PDF, Gases
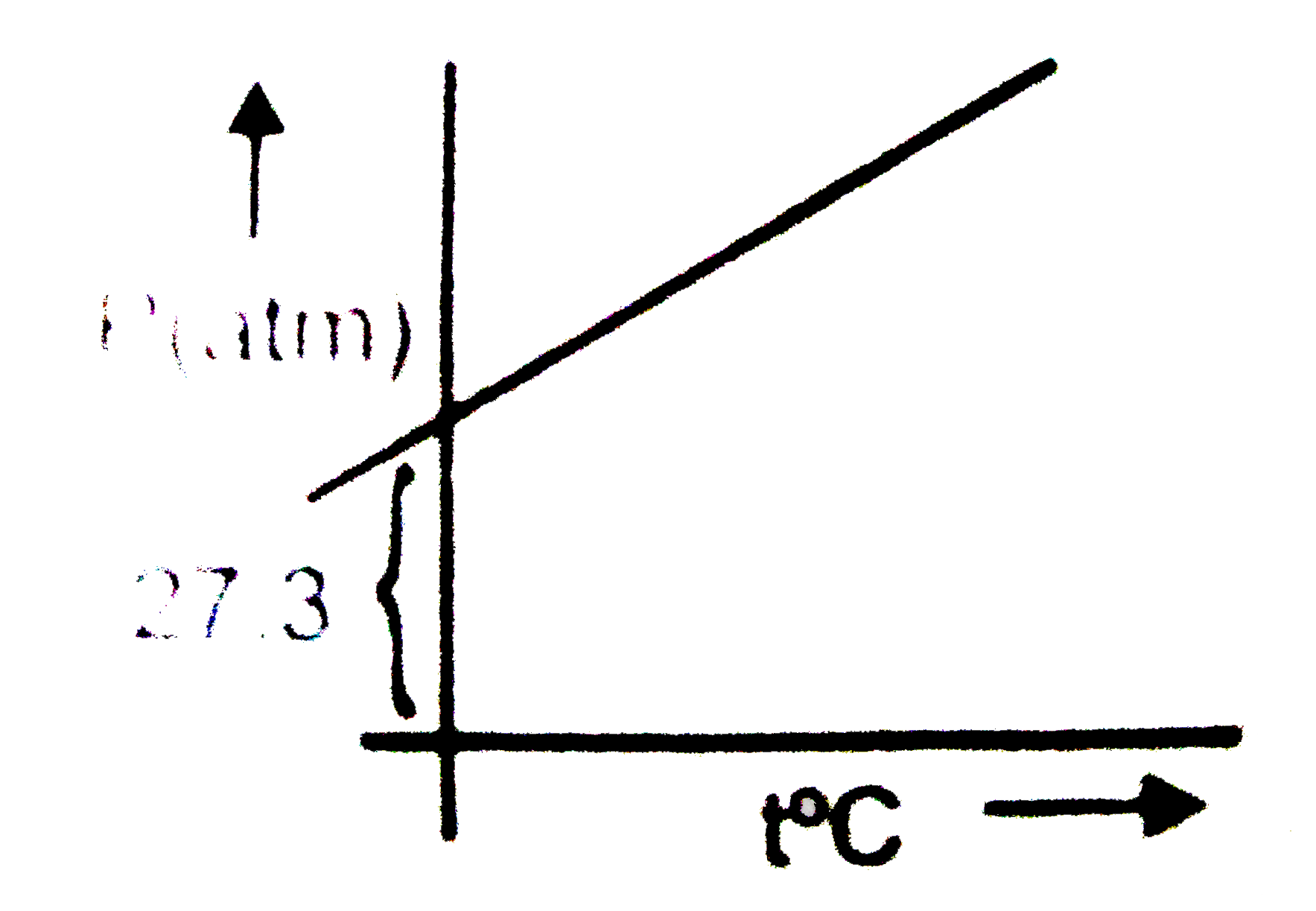
Level 1 (Q.31 To Q.60) from NARENDRA AWASTHI GASEOUS STATE for
- Non-Ideal Gas Behavior Chemistry: Atoms First

- Energies, Free Full-Text
- For $CO$, isotherm is of the type as shown. Near the point

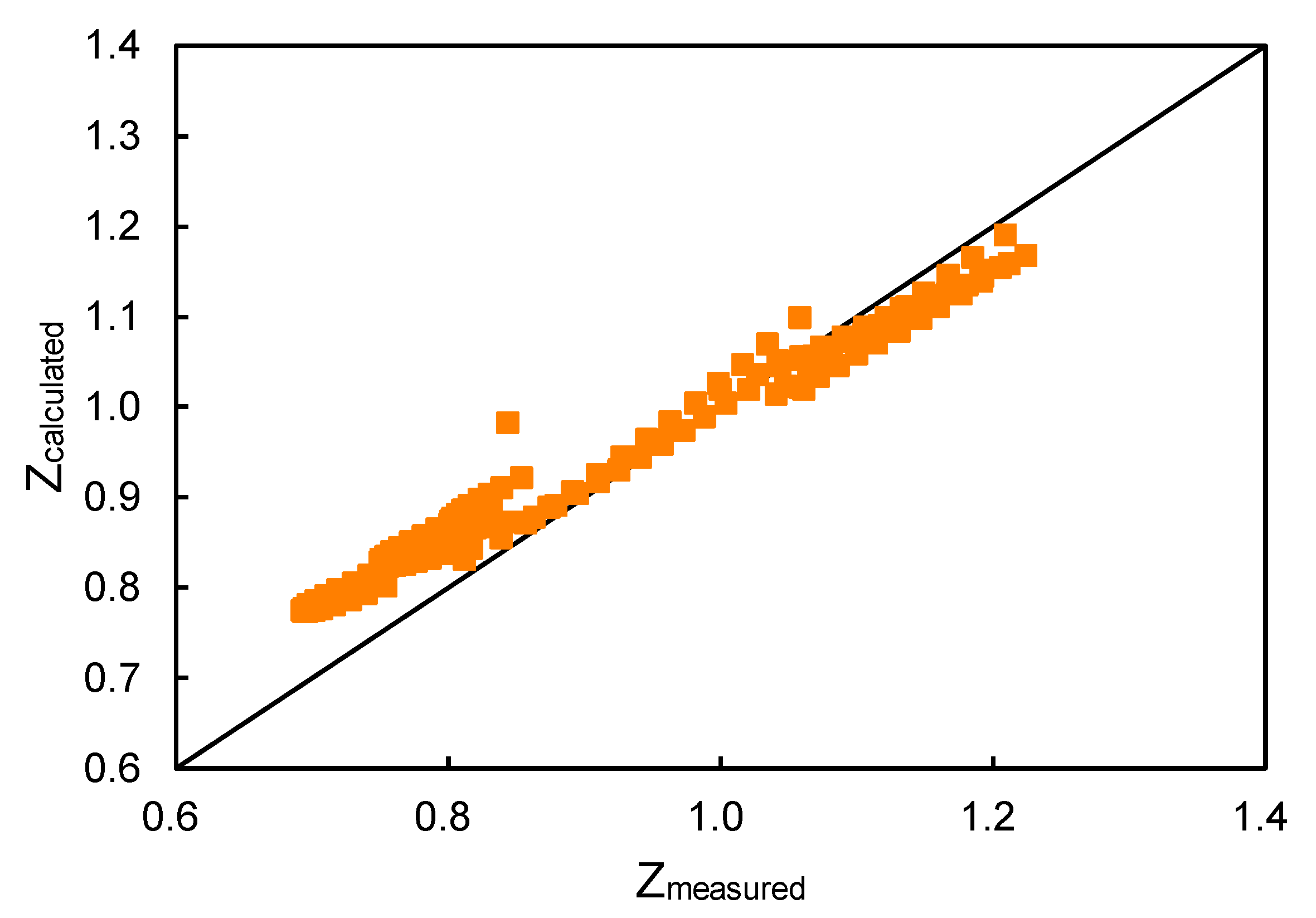
- PDF] Two Simple yet Accurate Equations for Calculating the Fugacity Coefficient Phi and the Gas Compressibility Factor

- For $CO$, isotherm is of the type as shown. Near the point compressibility factor $Z$ is?\n \n \n \n \n 1.$\\left( {1 + \\dfrac{b}{V}} \\right)$ 2.$\\left( {1 - \\dfrac{b}{V}} \\right)$3.$\\left( {1 + \\





